
[Science Talk] Should I switch to Stevia?
Over the past year, there has been a hype among the people around me about a "new" sweetener in the market. Probably due to the releases and promotions of Coca-Cola Life and Pepsi Next in Malaysia. And with the internet buzzing about it, my parents kept asking me, "What is stevia?" On the internet, it was claimed that the sweetener is better than regular sugar. This led me to ask, what makes it different from sugar? And another question popped up, what makes regular sugar so bad?
Okay, let's start from the beginning. What is Stevia? A quick search on the internet reveals that it is a sweetener extracted from the plant Stevia rebaudiana, or commonly known as candyleaf, sugarleaf, sweet leaf or sweetleaf. The plant is native to Paraguay and Brazil. The Guaraní people has been using the leafs for more than 1500 years (Wikipedia). They called the plant with various names; Caa-ehe, Azuca-caa, Kaa-he-e and Ca-a-yupe, all the names drew attention to the sweetness of the plant (Mowry, 1992).
In an article titled "Hints for the Dinner Table" by H. S. Fleming for The Decorator and Furnisher (vol. 12, no. 3) in 1888, it was written
Now, the next question is, "What makes it sweet?" I shall try to make this part simple. The active compounds in stevia are known as steviol glycosides. Brandle et al (1998) reported that the steviol glycosides are much sweeter than sucrose (a type of simple sugar), heat stable, pH stable and do not ferment. These glycosides are mainly made up of stevioside and rebaudiosides. Both compounds have glucose subgroups - this is the source of the sweet taste.
Essentially, the sweetness in stevia is caused by glucose molecules in the plant's active compounds. How is this different from regular sugar that we know?
Let's recall our Science lessons and understand what is sugar. Sugar, in simple words, is a sweet, short-chain carbohydrates, made up of carbon, hydrogen and oxygen. Commercial sugars (table sugars) are extracted and refined from sugar canes or sugar beets.
There are different types of sugar - simple sugars (monosaccharides) and compound sugars (disaccharides). Monosaccharides are sugars that are made up of single molecule, mostly with six carbon atoms, 12 hydrogen atoms and six oxygen atoms (C6H12O6), such as glucose, fructose and galactose. Compound sugars are combination of two molecules of simple sugars, mostly with general chemical formula C12H22O11 such as lactose, maltose and sucrose. Table sugars are made of refined sucrose.
In short, stevia is made up of sugar molecules and a non-sugar molecule (known as steviol). Are you confused yet? Alright... let's leave the chemistry lesson behind.
So why is stevia a better sweetener? To answer that question, first we have to look into how stevia is digested in the human body and compare it with the metabolism of table sugar (sucrose). Steviol glycosides are broken down into glucose and steviols. The steviols are not absorbed into the body and are secreted out, unchanged (Koyama et al, 2003). The glucose molecules released are used by the intestinal bacteria. Thus, they are not absorbed into the human body. Table sugar, on the other hand, are broken down into glucose and fructose (simple sugars). The glucose and fructose molecules are absorbed into the bloodstream (Gray, 1971). Therefore, stevia is a better sweetener and a perfect replacement of table sugar - especially for diabetic patients (Goyal et al, 2010). As glucose molecules in stevia are not absorbed by the human body, this allow the control of blood glucose level (Chen et al, 2005).
Despite all of these, I do believe that consumption of stevia should be exercised with caution. While diabetic patients benefit a lot from this, hypoglycemic effect of stevia may cause more harm on common people i.e. non-diabetic patients. In my opinion, stevia should only be taken as sweetener (in food or beverages) but it should not be replacing the carbohydrate in our diets. Even though there is no record of any side effects of stevia, there is also no formal long-term study on its effect either.
Will I switch table sugar to stevia anytime soon? No. There are plenty of reasons for this, but mainly because I think that it is currently overrated in Malaysia that causes the fluctuation of its price on the market in this country. Secondly, there are plenty of other sugar replacements - natural honey, for example. If you have time, maybe you'd like to read this out too, a second opinion in the sea of people that are telling you to switch to stevia.
P/s: Feel free to correct me, if I stated anything wrong.
 |
| Image taken from here through Google Image search |
In an article titled "Hints for the Dinner Table" by H. S. Fleming for The Decorator and Furnisher (vol. 12, no. 3) in 1888, it was written
At another dinner table, she filled a cut glass pitcher with sweet stevia and a few bon buds, and tying two or three yards of pale pink Marseilline silk through the pitcher's handle, let the ends drift along down the table.While this is not really a scientific observation or description of stevia, I think that it is worth noting that it has been recorded in the late 1800s - not as new as some people may thought. The first official scientific record was by Bertoni in 1899, where he described that a small piece of the leaf of the plant can sweeten the mouth for an hour. He observed the natives using the leaves to sweeten their tea and was amazed by the sweetness the leaves left. Bertoni brought the plant to the European's attention and was credited to the discovery of the species, ignoring the fact that it has been used by the Guaraní people for centuries. Despite his "discovery", there was limited research carried out on the plant. It was in 1930s that there was a formal scientific research carried out to study Stevia rebaudiana. Two French scientists extracted the chemical compounds that caused the sweet taste in the leaf (Bridel & Lavielle, 1931).
Now, the next question is, "What makes it sweet?" I shall try to make this part simple. The active compounds in stevia are known as steviol glycosides. Brandle et al (1998) reported that the steviol glycosides are much sweeter than sucrose (a type of simple sugar), heat stable, pH stable and do not ferment. These glycosides are mainly made up of stevioside and rebaudiosides. Both compounds have glucose subgroups - this is the source of the sweet taste.
 |
| Structural formula of stevioside. The blue compounds are glucose molecules, black compound is steviol. |
Let's recall our Science lessons and understand what is sugar. Sugar, in simple words, is a sweet, short-chain carbohydrates, made up of carbon, hydrogen and oxygen. Commercial sugars (table sugars) are extracted and refined from sugar canes or sugar beets.
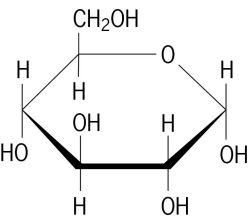 |
| Structural formula of glucose (Image from The Free Dictionary) |
In short, stevia is made up of sugar molecules and a non-sugar molecule (known as steviol). Are you confused yet? Alright... let's leave the chemistry lesson behind.
So why is stevia a better sweetener? To answer that question, first we have to look into how stevia is digested in the human body and compare it with the metabolism of table sugar (sucrose). Steviol glycosides are broken down into glucose and steviols. The steviols are not absorbed into the body and are secreted out, unchanged (Koyama et al, 2003). The glucose molecules released are used by the intestinal bacteria. Thus, they are not absorbed into the human body. Table sugar, on the other hand, are broken down into glucose and fructose (simple sugars). The glucose and fructose molecules are absorbed into the bloodstream (Gray, 1971). Therefore, stevia is a better sweetener and a perfect replacement of table sugar - especially for diabetic patients (Goyal et al, 2010). As glucose molecules in stevia are not absorbed by the human body, this allow the control of blood glucose level (Chen et al, 2005).
Despite all of these, I do believe that consumption of stevia should be exercised with caution. While diabetic patients benefit a lot from this, hypoglycemic effect of stevia may cause more harm on common people i.e. non-diabetic patients. In my opinion, stevia should only be taken as sweetener (in food or beverages) but it should not be replacing the carbohydrate in our diets. Even though there is no record of any side effects of stevia, there is also no formal long-term study on its effect either.
Will I switch table sugar to stevia anytime soon? No. There are plenty of reasons for this, but mainly because I think that it is currently overrated in Malaysia that causes the fluctuation of its price on the market in this country. Secondly, there are plenty of other sugar replacements - natural honey, for example. If you have time, maybe you'd like to read this out too, a second opinion in the sea of people that are telling you to switch to stevia.
P/s: Feel free to correct me, if I stated anything wrong.
